Photodynamics – the technology behind the DYPHOX process

Visible light
400–700nm
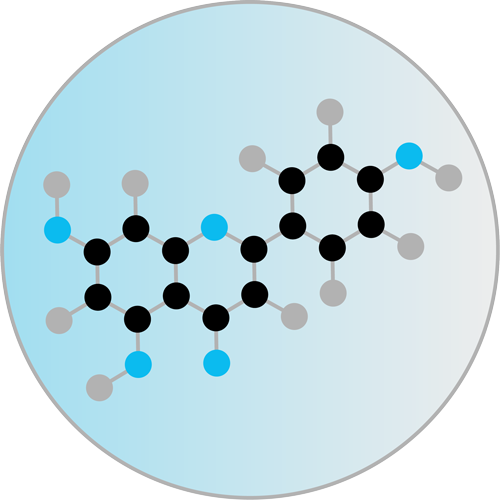
Activation of the
photocatalyst

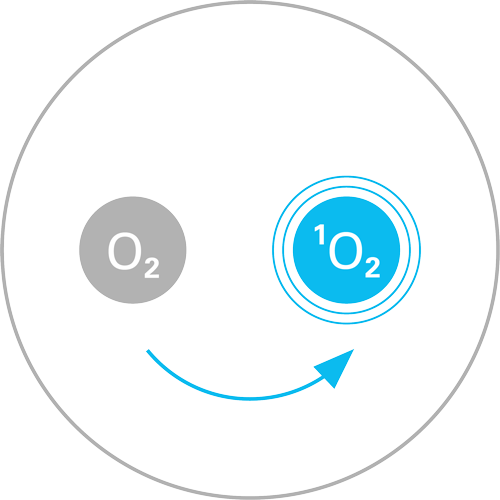
Production
of singlet oxygen
The antimicrobial effectiveness of DYPHOX is based on the principle of photodynamics, which was discovered by the German scientists Oskar Raab and Hermann von Tappeiner as early as the end of the 19th century. Photodynamics use special photocatalysts that absorb light in the visible light range (400–700 nm) and efficiently convert it into either oxygen radicals or singlet oxygen. The oxidative effect of these reactive oxygen species (ROS) can be used to efficiently kill bacteria, viruses, fungi, biofilms and spores. Photodynamics have been used successfully for years in cancer therapy, dentistry and ophthalmology, and in the disinfection of blood stocks.
By successfully modifying herbal and plant ingredients, we have identified photocatalysts that absorb light in the visible range (400–700 nm) and produce singlet oxygen with an efficiency of up to 99%. The range of the short-lived, gaseous singlet oxygen is about 8 mm, which is large enough to reach all germs on the surface, but also small enough not to be released uncontrolled into the surrounding space.
Antimicrobial photodynamics
Oxidative degeneration of Staphylococcus aureus

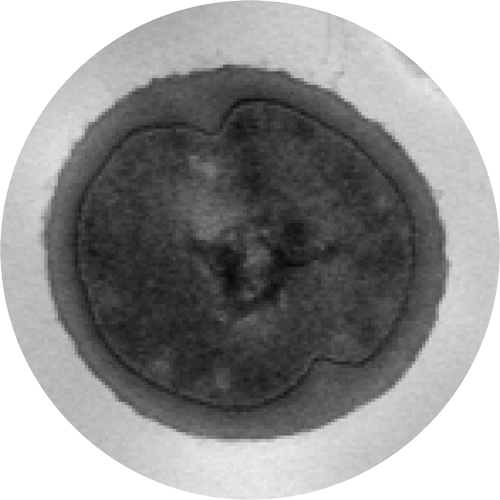
The Gram-positive, coccus-shaped bacterium Staphylococcus aureus is surrounded by a stable cell wall.

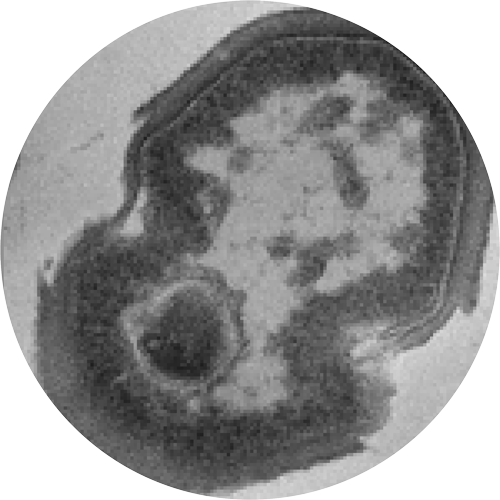
The photodynamic effect of DYPHOX technology causes rapid and efficient oxidative degeneration of the bacterial cell wall, efficiently killing the bacterium.
Singlet oxygen is a mild oxidising agent which on the one hand effectively kills germs. On the other, singlet oxygen, unlike other oxygen radicals, does not induce premature material ageing. Compared to other technologies, no moisture is required to effectively kill germs either. Therefore, DYPHOX is particularly suitable for the antimicrobial treatment of surfaces which are usually dry.
Moreover, there is no resistance to singlet oxygen known to date and there is not expected to be any, due to the non-specific mechanism of action. This is particularly important in light of the increase in multi-resistant bacterial strains in recent years.
Surface disinfection at a glance
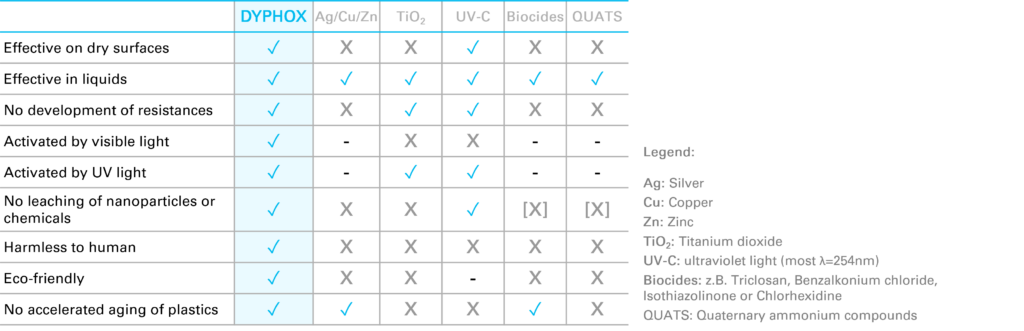
Comparison of methods for surface disinfection

Silver/copper/zinc-treated surface coatings
Silver, copper and zinc ions have antimicrobial properties. Silver is used, for example, in the antimicrobial treatment of wound dressings or bladder catheters. This makes sense because both are used in moist conditions. Only liquid enables the efficient transport of the ions to the germs and thus the antimicrobial effectiveness. Metal ions and particles are less suitable for surface application, as their effectiveness is very much reduced under dry conditions. Particles are also continuously released into the environment and there have been multiple reports of resistance development.

Titanium dioxide-treated surface coatings
The antimicrobial effectiveness of titanium dioxide is based on the compound’s photocatalytic effect. By activating titanium dioxide with UV-A (315–380nm) irradiation, radical oxygen species such as the hydroxyl and superoxide radical are formed. These reactive oxygen species (ROS) can efficiently inactivate germs. However, the higher reactivity of the radical oxygen species will also attack plastic surfaces over the long term. UV-A radiation also penetrates the tissue layers of the skin and the eye and damages them. Their use in surface technology is therefore only possible in accordance with safety conditions.
Surface disinfection with UV-C radiation
The antimicrobial effect of UV-C radiation is based on damage to the genetic information of bacteria and viruses. The high-energy, short-wave light induces strand breaks in the nucleic acid chains and thus causes a bacteriostatic or virustatic effect. UV-C irradiation can be used, for example, for the chemical-free disinfection of water systems. Similarly to the use of titanium dioxide, the use of UV-C radiation to disinfect surfaces is only possible under certain safety conditions.

Surface disinfection using biocides
The antimicrobial effect of biocides such as benzalkonium chloride, triclosan, isothiazolinones or chlorhexidine comes from “poisoning” the germs. Any concentration of these toxic substances in the ecosystem or human body should be avoided as far as possible. The permanent treatment of surfaces with classical biocides is therefore concerning. There is also only limited effectiveness on dry surfaces. The RKI also strongly advises against the widespread use of biocidal agents such as triclosan, chlorhexidine and benzalkonium chloride, as clinically relevant germs have been shown to develop strong and stable resistance to these agents. The development of cross-resistance to antibiotics is also well known and very worrying.
